Learning objectives
|
Unlike CT, which requires ionising radiation, MRI is based on the
interaction between radio waves and hydrogen nuclei in the body in the
presence of a strong magnetic field. There is no ionising radiation and
no known safety issues. MRI produces higher quality images than CT. In
CT scans must be in the plane of the gantry, that is, axial or
semi-coronal. In MRI, one is able to acquire images directly in any
plane, that is, the usual axial, sagittal, coronal, or any other.
Both modalities focus on the properties of a volume element or
"voxel" of tissue. This is represented in 2-D form or picture elements
or "pixels". The pixel intensity in CT reflects the electron density
but in MRI it reflects the density of hydrogen, generally as water (H20)
or fat. To be more exact, MR signal intensity reflects the density of
mobile hydrogen nuclei modified by the chemical environment, that is,
by the magnetic relaxation times, T1 and T2, and by motion.
A hydrogen nucleus is a single proton. It has a positive charge
and spins and so generates a small magnetic field (a "magnetic moment")
which are usually randomly distributed. These magnetic moments align
when placed in a larger magnetic field. With MRI the magnetic field
across the body-sized sample is intentionally made non-uniform by
superimposing additional magnetic field gradients that can be turned on
and off rapidly. Activation of these additional magnetic fields results
in a net gradient in the strength of the magnetic field across the body
which is necessary for spatial localisation and imaging. The essential
components of an MR imaging system include a large magnet which
generates a uniform magnetic field, smaller electromagnetic coils to
generate magnetic field gradients for imaging and a radio transmitter
and receiver and its associated transmitting and receiving antennae or
coils. In addition to these fundamental components, a computer is
necessary to coordinate signal generation and acquisition and image
formation and display.
When the body lies within a strong magnetic field, it becomes
temporarily magnetised. This state is achieved when the hydrogen nuclei
in the body align with the magnetic field. When magnetised, the body
responds to exposure to radio waves at a particular frequency by sending
back a radio wave signal called a "spin echo". This phenomenon (NMR)
only occurs at one frequency (the "Larmor frequency") corresponding to
the specific strength of the magnetic field. The spin echo signal is
composed of multiple frequencies, reflecting different positions along
the magnetic field gradient. When the signal is broken into its
component frequencies (by a technique called a "Fourier Transform"),
the magnitude of the signal at each frequency is proportional to the
hydrogen density at that location, thus allowing an image to be
constructed. Thus, spatial information in MRI is contained in the
frequency of the signal, unlike X-ray-based imaging modalities such as
CT.
Brain MRI usually involves lying within a very tight space for between 10 and 30 minutes. Access to the patient is difficult. It is incredibly noisy and communication with radiographers is difficult. One has to be realistic and consider the shortest protocol that will get the important clinical information needed. Some do not tolerate it at all. It is a common paradox and frustration that those in whom it would be of most value can't have one due to one reason or other. There are some open scanners available for those who are claustrophobic but access is often difficult.
Metal outside the brain and eye is NOT an absolute contraindication: Magnetic deflection is minimal compared to normal physiologic forces. Cardiac valves), inferior vena cava filters, biliary and vascular stents, IUD's and metallic prostheses are safe, unless there is doubt as to positional stability. MRI Policies - Safety and Contraindications . Information is changing all the time. Another useful site is MRI safety.com
This is the MRI equivalent of CT with contrast and uses Gadolinium
which shortens T1 relaxation times. It is useful when there is
suspicion of neoplastic, inflammatory lesions or abscesses. Also useful
for detecting meningeal disease. It is not often used in acute stroke
unless the diagnosis is in doubt.
MRI Basics
Sometimes MRI is not possible
Reasons why MRI not possible
Examples
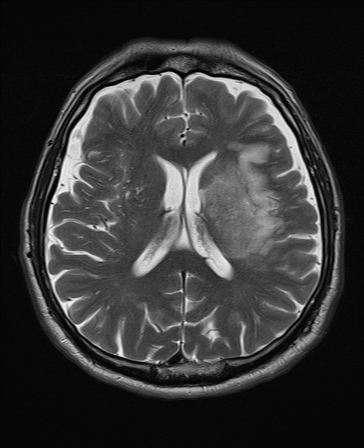
T2 FLAIR T2 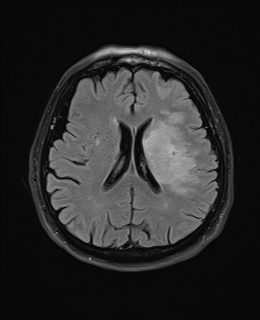
DWI ADC 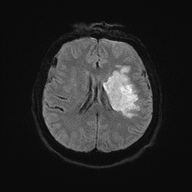

SWI T1 Sagittal 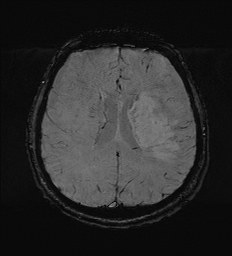
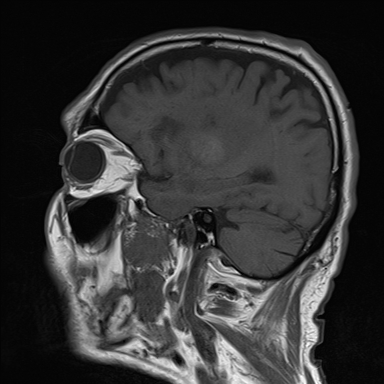
MRI with Gadolinium
MR Angiography
Scan Sequences
Relaxation times
- T1 relaxation time: Time taken for 63% of longitudinal magnetisation to recover in a tissue. T1 is short in fat and long in water and proteins. Contrast causes T1 shortening.
- T2 relaxation time: Time taken for 63% of transverse magnetisation to be lost in a tissue. Liquids have long T2 and large molecules a short T2.
- T2* based on T2 decay and dephasing due to inhomogeneities in the magnetic field. Relevant in Gradient echo imaging.
- Weighting T1 and T2 weighting can increase tissue contrast
T1 weighted
- Scanning parameters are set (short TR/short TE)
- Dark : CSF and anything with increased water (oedema, tumour, infection, infraction, haemorrhage or flow void or calcification).
- Bright: Fat, subacute haemorrhage, melanin, protein rich fluid, slow flowing blood, gadolinium, laminar necrosis of an infarct. White matter is brighter than grey. Myelin is light grey. Grey matter is grey. T1 is better for showing anatomy. An acute stroke will be hypointense.
T2 Weighted
- Dark: Calcification, Blood products, protein rich fluid, flow void.
- Bright: Anything with increased water e.g. CSF, Oedema, tumour, Infarct, inflammation, infection, subdural collection, methaemoglobin in subacute bleed. CSF is white and brain tissue is darker and more intermediate. Fat is dark. Myelin is dark grey. Grey matter is brighter than white matter. Good for identifying pathology. T2 is bright due to water or any oedema in pathology. For example a fresh infarct with oedema will show up bright or hyperintense after a few days becoming most obvious after a few months. T2 weighted hyperintensities may be seen within 6 hours and are present in 90% by 24 hours.
T2* "star"
- T2* can be recorded using GRE sequences with a low flip angle, long TE, and long TR
- Mostly used to detect iron in its various forms. Haemorrhages cause an influx of haemoglobin and hemosiderin into the lesion.
- The iron in haemoglobin and haemosiderin is paramagnetic and thus causes an susceptibility difference in the damaged region, which shows up as dark regions in T2* scans.
- Blood looks black and Hemosiderosis can also be detected by T2* scans for the same reason.
- Calcification shows up bright in a T2* MRI due to its higher diamagnetism than the surrounding tissue.
FLAIR
- Fluid attenuated inverse recovery.
- FLAIR scans are T2 scans with the free water signal nulled.
- CSF is now dark. Useful to see oedema and periventricular lesions which appear bright.
- An acute ischaemic stroke will be bright
Gradient echo (GRE)
- Excellent at identifying areas of blood and haemosiderin deposition such as in macrophages around an old bleed.
- Also useful in identifying microbleeds. Sensitivity for blood approaches CT within first 24 hours of a bleed.
- After several days GRE is the more sensitive modality.
Diffusion Weighted Imaging
- Identifies areas where Brownian motion is restricted due to cytotoxic cell death. Useful for identifying early Ischaemic stroke.
- This is explained by loss of ATP causes of ion exchange pumps.
- Water from the extracellular space enters into the intracellular compartment (cytotoxic oedema) and produces a typical bright spot on DWI.
- These changes are seen even within 30 minutes. A new stroke is bright, but an old stroke will have low signal intensity on DWI.
- The DWI is initially bright white and then gradually fades after 10-15 days when the lesion will be best seen on T2 and FLAIR.
- The ADC often shows the inverse and is black.
- The signal intensity of acute stroke on DW images increases during the 1st week after symptom onset and decreases thereafter.
- The ADC map shows a similar findings.
| Restricted diffusion: Bright DWI and Dark ADC |
|---|
|
| Left MCA infarct | DWI of an Anterior cerebral artery infarct |
|---|---|
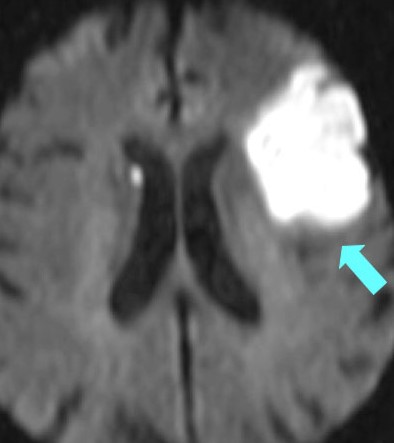 | 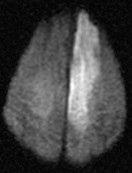 |
Apparent Diffusion Coefficient: (ADC map)
Used with DWI. Ischaemic lesions appear dark. If bright this may suggests the DWI increased signal changes are due to T2 shine through and old i.e. false positive.
Gadolinium Enhancement
Identifies pathology in which there is breakdown of the blood brain barrier. Also useful in producing an angiogram. Tumours or other lesions may show ring like enhancement. T1 with Gadolinium will show increased signal with a pituitary tumour, acoustic neuroma or meningioma.
Susceptibility weighted imaging (SWI)
SWI is an MRI sequence sensitive to paramagnetic compounds which distort the local magnetic field. It can detect blood, iron and calcium etc. and so is useful to detect haemorrhage or blood. Images generate a unique contrast, different from that of spin density, T1, T2, and T2*. It is very sensitive over other sequences. It is not possible to differentiate calcium from blood. A filtered phase can allow distinguish between the two as diamagnetic and paramagnetic compounds will affect phase differently (i.e. veins / haemorrhage and calcification will appear of opposite signal intensity). SWI is very useful in detecting cerebral microbleeds in ageing and occult low-flow vascular malformations, in characterising brain tumours and degenerative diseases of the brain, and in recognizing calcifications in various pathological conditions. The phase images are especially useful in differentiating between paramagnetic susceptibility effects of blood and diamagnetic effects of calcium. SWI can also be used to evaluate changes in iron content in different neurodegenerative disorders. Reference

MRI Imaging
| Acute Ischaemic Stroke |
|
| Cardioembolic stroke |
|
| Lacunar Stroke |
|
| Basilar Artery Occlusion |
|
| Carotid Dissection |
|
| Cerebral/Vertebral venous sinus thrombosis |
|
| CADASIL |
|
| Primary angiitis of the CNS |
|
| MELAS syndrome |
|
| Posterior reversible encephalopathy syndrome (PRES) |
|
Imaging Patterns in Haemorrhagic Stroke
| Hypertensive haemorrhage |
|
| Cerebral Amyloid Angiopathy |
|
Other relevant imaging Diagnoses
Non Communicating Hydrocephalus
Radiology of choice is Midsagittal MRI which reveals the drainage pathway in great detail
- Normal - Lateral ventricles small and 3rd ventricle barely visible
- Single lateral ventricle dilated (Univentricular hydrocephalus) = Obstruction of one foramen of Monro
- Both lateral ventricles dilated (Biventricular) = Obstruction of both foramen of Monro
- Dilation of Lateral + 3rd Ventricle (Triventricular) = Obstruction at level of aqueduct
- Dilation of Lateral + 3rd + 4th = Obstruction at foramen of Magendie and Luschka
Communicating Hydrocephalus
- All ventricles are modestly dilated.
- Prominent Subarachnoid spaces and basal cisterns