Learning objectives
|
Introduction and Evidence Base
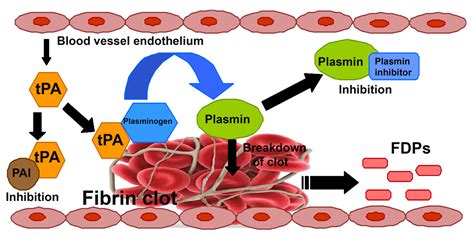
- Thrombolysis in its simplest terms means the enzymic breakdown of fibrin which forms the structure of an occlusive thrombus which then allows reperfusion.
- However unlike myocardial cells brain cells tolerate hypoxia and ischaemia far less and cell death occurs within minutes if flow falls below critical.
- Thrombolysis can never stop strokes or prevent infarction as cell death occurs very quickly within minutes but the idea is to reduce stroke size based on the concept of the ischaemic penumbra which is the volume of brain tissue that exists around the core infarct which exists between cell death and cell recovery and which can be rescued if reperfusion occurs.
| Stroke thrombolysis given IV will lead to recanalisation of proximal arterial occlusions in only 33% of patients. Mechanical thrombectomy provides much higher rates of recanalisation |
|---|
Thrombolytic Drugs
Alteplase (Actilyse) is currently the only drug licensed to treat Acute Stroke. Actilyse is a plasminogen activator. Once activated plamsinogen converts to plamsin and enzymically breaks down fibrin. Unlike with cardiac thrombolysis streptokinase has been found to be harmful in any study involving acute stroke. Other trials are looking at newer forms of t-PA but currently Alteplase is the only choice.
Evidence Base
In the light of the success of the use of thrombolysis in reducing mortality in cardiovascular trials in ST-elevation MI (and its dramatic lack of effect in NSTEMI)it was thought that a similar therapeutic benefit might be had with acute ischaemic stroke. Initial trials in stroke however were dramatically unsuccessful. This involved Streptokinase which in STEMIs was almost as good as Alteplase. It wasn't until NINDS trial that things took off and Alteplase was rapidly given FDA approval for use.
NINDS study
Thrombolytic therapy for acute ischaemic stroke up until the NINDS study had been approached cautiously because there were high rates of intracerebral haemorrhage in early clinical trials. In the NINDS study a randomised, double-blind trial of intravenous recombinant tissue plasminogen activator (t-PA) for ischaemic stroke was begun within three hours of the onset of stroke. The trial had two parts. Part 1 (in which 291 patients were enrolled) tested whether t-PA had clinical activity, as indicated by an improvement of 4 points over base-line values in the score of the National Institutes of Health stroke scale (NIHSS) or the resolution of the neurologic deficit within 24 hours of the onset of stroke. Part 2 (in which 333 patients were enrolled) used a global test statistic to assess clinical outcome at three months, according to scores on the Barthel index, modified Rankin scale, Glasgow outcome scale, and NIHSS.
In part 1, there was no significant difference between the group given t-PA and that given placebo in the percentages of patients with neurological improvement at 24 hours, although a benefit was observed for the t-PA group at three months for all four outcome measures. In part 2, the long-term clinical benefit of t-PA predicted by the results of part 1 was confirmed (global odds ratio for a favourable outcome, 1.7; 95 per cent confidence interval, 1.2 to 2.6). As compared with patients given placebo, patients treated with t-PA were at least 30 per cent more likely to have minimal or no disability at three months on the assessment scales. Symptomatic intracerebral haemorrhage within 36 hours after the onset of stroke occurred in 6.4 per cent of patients given t-PA but only 0.6 per cent of patients given placebo (P <0.001). Mortality at three months was 17 per cent in the t-PA group and 21 per cent in the placebo group (P = 0.30).
The trial was quickly followed by FDA approval for the use of alteplase. Many felt that the evidence base was not sufficient to support the widespread adoption of thrombolysis and these doubts lead to a slow take-up particularly in the UK as well as the logistical and organisational changes to support such an intensive and early therapy. Within the UK there were early adopters who built up thrombolysis services usually in well-resourced teaching hospitals with neuroscience centres interested in vascular neurology. Others were based on key individuals leading a rather onerous service. Alteplase received its European license for acute stroke in 2004 and the current license only covers its use in those within 3 hours of acute stroke. There are several other criteria
In acute ischaemic stroke alteplase is licensed subject to use in an appropriate clinical environment by a physician trained and experienced in neurological care. Treatment units must be registered with SITS-MOST (Safe Implementation of Thrombolysis in Stroke Monitoring System), an on-line database established in Sweden to collect information on patient outcomes when alteplase is used in routine practice. Those thrombolysed outside the 3-hour window are done off license a point that should be made clear.
NINDS demonstrated that thrombolysis within 3 hours of stroke onset improved outcome by reduced combined endpoints of death and disability
ECASS trial
ECASS (European Cooperative Acute Stroke Study) involved 620 patients given 1.1 mg/kg of Alteplase or placebo within 6 hours. Outcome showed a small benefit for those treated with alteplase but at the cost of extra early deaths.
ECASS 2 trial
ECASS 2 (European Cooperative Acute Stroke Study) randomised 820 patients to Alteplase 0.9 mg/kg or placebo. Strokes were less severe than ECASS. There was a very small benefit for thrombolysed patients.
ATLANTIS
In 2002 the ATLANTIS data was released on patients treated with alteplase within 3 hours of stroke onset. In total 61 patients enrolled in the Alteplase Thrombolysis for Acute Non-interventional Therapy in Ischaemic Stroke (ATLANTIS) study randomised to receive intravenous tPA or placebo within 3 hours of symptom onset. Despite a significant increase in the rate of symptomatic intracranial haemorrhage, tPA-treated patients were more likely to have a very favourable outcome (score of < or = 1) on the National Institutes of Health Stroke Scale at 90 days (P=0.01). These data supported the NINDS based recommendations to administer intravenous tPA to eligible ischaemic stroke patients who can be treated within 3 hours of symptom onset.
ECASS 3 trial
More positive evidence for the benefits of thrombolysis came out in the ECASS 3 trial in 2008 which extended the window beyond 3 hours to 4.5 hours after onset of stroke. Patients again were given alteplase after exclusion of brain haemorrhage or major infarction, as detected on a CT. Patients were randomly assigned in a 1:1 double-blind fashion to receive treatment with intravenous alteplase (0.9 mg per kilogram of body weight) or placebo. The primary endpoint was disability at 90 days, dichotomized as a favourable outcome (a score of 0 or 1 on the modified Rankin scale, which has a range of 0 to 6, with 0 indicating no symptoms at all and 6 indicating death) or an unfavourable outcome (a score of 2 to 6 on the modified Rankin scale). The secondary endpoint was a global outcome analysis of four neurologic and disability scores combined. Safety endpoints included death, symptomatic intracranial haemorrhage, and other serious adverse events.. In total 821 patients were enrolled in the study and randomly assigned 418 to the alteplase group and 403 to the placebo group. The median time for the administration of alteplase was 3 hours 59 minutes. More patients had a favourable outcome with alteplase than with placebo (52.4% vs. 45.2%; odds ratio, 1.34; 95% confidence interval [CI], 1.02 to 1.76; P=0.04). In the global analysis, the outcome was also improved with alteplase as compared with placebo (odds ratio, 1.28; 95% CI, 1.00 to 1.65; P<0.05). The incidence of intracranial haemorrhage was higher with alteplase than with placebo (for any intracranial haemorrhage, 27.0% vs. 17.6%; P=0.001; for symptomatic intracranial haemorrhage, 2.4% vs. 0.2%; P=0.008). Mortality did not differ significantly between the alteplase and placebo groups (7.7% and 8.4%, respectively; P=0.68). There was no significant difference in the rate of other serious adverse events.
As compared with placebo, intravenous alteplase administered between 3 and 4.5 hours after the onset of symptoms significantly improved clinical outcomes in patients with acute ischaemic stroke; alteplase was more frequently associated with symptomatic intracranial haemorrhage. Again alteplase did not alter mortality but did improve outcome. In terms of effectiveness this has been looked at. Analysis post-ECASS 3 showed that treatment with tissue plasminogen activator in the 3- to 4.5-hour window confers benefit on approximately half as many patients as treatment < 3 hours, with no increase in the conferral of harm. Approximately 1 in 6 patients has a better and 1 in 35 has a worse outcome as a result of therapy. We are currently awaiting the results of the IST 3 trial which again has pushed the limits to those over the age of 80 and beyond the 3-hour mark.
ECASS 3 showed that thrombolysis times could be extended out to 4.5 hours post stroke onset though less effective than earlier administration
Benefits of Thrombolysis
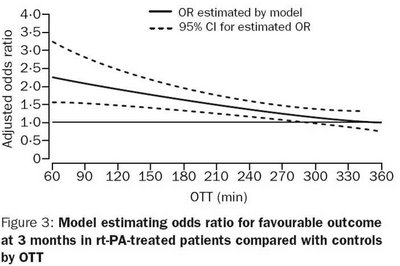
The graph above shows a clear fall off in benefit with time. One can separate patients into different cohorts - the first 90 minutes when benefits are maximal so one may be keener to treat, the 90-180 minutes where there are still gains and the final 180 mins out to 270 minutes where benefits are rapidly diminishing and so the risk should be reduced commensurately.
IST 3 Trial
This data has only been available since May 2012. This randomised control trial data has shown that it is safe to treat older people. The third International Stroke Trial (IST-3) sought to determine whether a wider range of patients might benefit up to 6 h from stroke onset. For the types of patient recruited in IST-3, despite the early hazards, thrombolysis within 6 h improved functional outcome. Benefit did not seem to be diminished in elderly patients. For every 1000 patients given rt-PA within three hours of a stroke, 80 more will survive and live without help from others than if they had not been given the drug. There is an early risk of death in the first week due to symptomatic intracranial haemorrhage.
IST-3 data showed that there was no upper age limit cut off for the administration of thrombolysis
SITS Observational database findings
The chart shows the response to treatment of each group measured as their Modified Rankin score
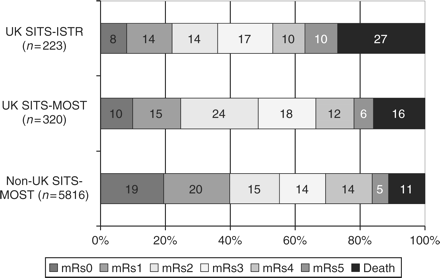
[Lees KR et al. 2008]
Practical aspects of Administering Alteplase
Consenting for Thrombolysis
Consenting patients for thrombolysis can be difficult. Benefits are constantly falling rapidly with time. The risk of symptomatic ICH is small but real about 5-6%. There are also risks of other complications although rare. Patients are often dysphasic or if not dysphasic are anxious and in a strange and frightening environment. they have just been brought in, had a CT scan and most have no idea what a stroke is. It is really not an environment that one should ever be asked to make a personal decision. One would never make a life-changing decision with so little knowledge or time.
It is hard to regard the consenting process as being fully informed as the patient is in no state really to take in and process information. However, time is not available for the patient to take long to consider the risks and benefits. Most patients have seen the adverts on the TV and there is an inherent trust in that that certainly helps the patient to accept the treatment. It must be made clear that there are real risks and the treatment may lead to disability and death but that it is more likely to reduce these.
Where the patient is not able to consent such as due to severe dysphasia then one must act in the best interests of the patient. If the family are available it is wise to get them on side and explain to them what is happening but they have no real legal standing unless they hold a lasting power of attorney to manage the patient's affairs when they become incapacitated. Family can, however, communicate the patients’ views if any have been expressed before - a sort of decision by proxy.
There is a symptomatic bleed risk of about 5% however that is not associated with increased mortality in those receiving thrombolysis. The most recent data suggest that treating 100 patients will result in an additional 8 people being alive or with minimal disability than would otherwise be. Generally with thrombolysis most of us accept verbal consent as that is quicker.
Where consent is not possible due to a communication issue e.g. dysphasic patient then the doctor may act in the patients best interests. This is not to give the doctor the right to override a patients decision which the doctor may regard as unwise. The medical professional must act sensibly and with the patient's best interests foremost and should certainly be able to justify their actions to a court, or their lack of action. A patient may later challenge a decision in which they were denied treatment. Ultimately the responsibility rests on the consultant in charge.
NOTE THIS CAN ONLY BE DONE BY OR UNDER THE DIRECT SUPERVISION OF SOMEONE FORMALLY TRAINED IN STROKE THROMBOLYSIS. CHECK YOUR HOSPITAL PROTOCOL
A very user-friendly proforma makes the process safer and faster. Go through it line by line.
Before giving Alteplase check that..
| INCLUSION CRITERIA - ALL MUST BE NO TO THROMBOLYSE | CHECK ALL NO |
|---|---|
| Is the GCS < 9 ? | MUST BE NO |
| Are there rapidly resolving symptoms ? | MUST BE NO |
| Is other pathology likely (e.g. brain tumour or septic embolus etc) ? | MUST BE NO |
| Is there Uncontrolled hypertension (systolic >185mg or diastolic >110mg) even despite treatment ? | MUST BE NO |
| Is the NIH Score < 4 or >=25 (caution >22) ? | MUST BE NO |
| Is there Fixed head or eye deviation | MUST BE NO |
Do not proceed with thrombolysis assessment if any of the above answers are YES - take advice if unsure.
| EXCLUSION CRITERIA ALL MUST BE NO TO THROMBOLYSE | CHECK ALL NO |
|---|---|
| Seizure at presentation onset | MUST BE NO |
| Suspected SAH even if CT normal (It would seem unusual to have a NIHSS in the treatment range and no blood on CT. Always look carefully in cisterns and posterior aspect of lateral ventricle. I am more worried about convexity sulcal bleeds which can give mild neurological loss and may be missed on CT and may not be associated with headache and more related to CAA or RCVS) | MUST BE NO |
| Recent puncture of a artery or non-compressible blood-vessel (e.g. subclavian or jugular vein puncture) or lumbar puncture within 7 days. | MUST BE NO |
| Traumatic cardiopulmonary resuscitation less than 10 days. | MUST BE NO |
| Surgery or visceral biopsy within previous 4 weeks | MUST BE NO |
| History of recent bleeding (where from, amount, significance and could it be controlled) | MUST BE NO |
| Pregnant or could they be or childbirth within the previous 4 weeks | MUST BE NO |
| Breastfeeding - can treat if agrees to stop breastfeeding temporarily | MUST BE NO |
| Head injury at the time of stroke or in past 3 months. | MUST BE NO |
| Significant trauma (fracture or internal injuries) in past 3 months | MUST BE NO |
| Major surgery in past 3 months (could we control bleeding if it occurred) | MUST BE NO |
| Ischaemic Stroke within the last 3 months. | MUST BE NO |
| Haemorrhagic stroke at any time in the past | MUST BE NO |
| Documented ulcerative gastrointestinal disease during the last 3 months | MUST BE NO |
| Oesophageal varices, Active peptic ulcer disease, Severe liver disease including hepatic failure, Cirrhosis. | MUST BE NO |
| Portal hypertension (oesophageal varices) and active hepatitis, Pancreatitis. | MUST BE NO |
| Neoplasm with increased bleeding risk | MUST BE NO |
| Intracranial neoplasm, Arteriovenous malformation or aneurysm | MUST BE NO |
| Endocarditis, Pericarditis, Arterial-aneurysm | MUST BE NO |
| Arterial/venous malformations aortic aneurysm or ventricular aneurysm | MUST BE NO |
| Any known bleeding problem/ blood disorder | MUST BE NO |
| Haemorrhagic retinopathy (e.g. untreated proliferative diabetic retinopathy) | MUST BE NO |
| History of any past stroke and diabetes mellitus* | MUST BE NO |
| On warfarin (INR>1.4) or equivalent anticoagulation | MUST BE NO |
| Administration of heparin or equivalent within the previous 48 hours and an APTT exceeding the upper limit of normal for laboratory | MUST BE NO |
* - discuss as this is regarded as a relative contraindication
Do not proceed with thrombolysis assessment if any of the above answers are YES - take advice if unsure especially if borderline. Some may thrombolyse to an INR of 1.5
| CT FINDINGS - ALL MUST BE NO TO THROMBOLYSE | Check all NO |
|---|---|
| CT Hypodensity or sulcal effacement in >1/3 of MCA territory | MUST BE NO |
| Evidence of Haemorrhage, tumour, abscess or developed stroke | MUST BE NO |
| Evidence of Arteriovenous Malformation or Aneurysm | MUST BE NO |
| CT evidence of old stroke in a patient with diabetes | MUST BE NO |
| Major early infarct signs on a CT scan (substantial oedema, mass effect or midline shift) | MUST BE NO |
Difficult Cases : Advice from AHA Guidelines 2018
| Concerns | Comments |
|---|---|
| Microbleeds | In otherwise eligible patients who have had a previously demonstrated small number (1-10) of CMBs on MRI, administration of IV alteplase is reasonable. In otherwise eligible patients who have had a previously demonstrated high burden of CMBs (>10) on MRI, treatment with IV alteplase may be associated with an increased risk of sICH, and the benefits of treatment are uncertain. Treatment may be reasonable if there is the potential for substantial benefit. |
| Sickle cell | IV alteplase for adults presenting with an AIS with known sickle cell disease can be beneficial. |
| LMWH | IV alteplase should not be administered to patients who have received a treatment dose of low-molecular-weight heparin (LMWH) within the previous 24 hours. |
| Menstruation | IV alteplase is probably indicated in women who are menstruating who present with AIS and do not have a history of menorrhagia. However, women should be warned that alteplase treatment could increase the degree of menstrual flow. Because the potential benefits of IV alteplase probably outweigh the risks of serious bleeding in patients with recent or active history of menorrhagia without clinically significant anaemia or hypotension, IV alteplase administration may be considered |
| Unruptured Saccular aneurysm | For patients presenting with AIS who are known to harbour a small or moderate-sized (<10 mm) unruptured and unsecured intracranial aneurysm, administration of IV alteplase is reasonable and probably recommended.†(Class IIa; LOE C-LD)‡ |
| Pregnancy | IV alteplase administration may be considered in pregnancy when the anticipated benefits of treating moderate or severe stroke outweigh the anticipated increased risks of uterine bleeding.†(Class IIb; LOE C-LD)‡ |
| Post Partum | The safety and efficacy of IV alteplase in the early postpartum period (<14 d after delivery) have not been well established.†(Class IIb; LOE C-LD)‡ |
| Ophthalmological conditions | Use of IV alteplase in patients presenting with AIS who have a history of diabetic haemorrhagic retinopathy or other haemorrhagic ophthalmic conditions is reasonable to recommend, but the potential increased risk of visual loss |
Do not proceed with thrombolysis assessment if any of the above answers are YES - take advice if unsure.
| INCLUSION CRITERIA - ALL MUST BE YES TO THROMBOLYSE | CHECK ALL YES |
|---|---|
| CT compatible with acute ischaemic stroke or normal. | MUST BE YES |
| Thrombolysis can be given within 4.5 hrs of stroke onset (new update to Alteplase licence) licence protocol) | MUST BE YES |
| Patient Age 18-80 (Older patients should be considered but off licence) | MUST BE YES |
| Patient is independent | MUST BE YES |
| Verbal or written informed consent or assent available | MUST BE YES |
| NIHSS ≤ 22 | MUST BE YES |
| BP is < 185/110 mmHg and confident you can maintain it below this level. | MUST BE YES |
Do not proceed with thrombolysis assessment if any of the above answers are NO - take advice if unsure.
Lowering Blood pressure before thrombolysis
Blood pressure must be below 185/110 mm Hg for thrombolysis and maintained below that level for the first 12 hours. If over 185/110. BP may be treated with a nitrate patch or nitroglycerin paste and/or one or two 10-20 mg doses of LABETALOL given IV push within one hour. Practically if the patient can take a beta blocker I prefer the ease of giving Labetalol.If these measures do not reduce BP below 185/110 and keep it down, the patient should not be treated with rt-PA.
Test results
| CONTRAINDICATIONS TO LYSIS IF DONE: ALL NEED A BM CHECKED | CHECK ALL NO |
|---|---|
| Blood glucose <3 mmols/l or >22 mmols/l despite treatment | MUST BE NO |
| Platelet count < 100 x 109/l | MUST BE NO |
| Hb < 10 g/dl or Haematocr it <25% - If there is no clinical reason whatsoever to suspect an abnormal result thrombolysis should not be delayed to wait for the results. FBC can usually be done in A&E analyser | MUST BE NO |
| Abnormal INR >1.4 or APTT > 36 seconds - If there is no clinical reason whatsoever to suspect an abnormal FBC or INR thrombolysis should not be delayed to wait for the results | MUST BE NO |
STOP NOW AND REASSESS BEFORE OPENING AND GIVING ALTEPLASE

| FINAL CHECK | CHECK ALL YES |
|---|---|
| Is stroke the most likely diagnosis | MUST BE YES |
| Is the patient being treated within 4.5 hours of stroke onset | MUST BE YES |
| Are inclusion criteria all true | MUST BE YES |
| Are all exclusion criteria false | MUST BE YES |
| Is the CT compatible with ischaemic stroke | MUST BE YES |
| Is the BP < 185/110 mmHg and can you confidently maintain it below this level | MUST BE YES |
| Is the NIHSS ≤ 22 | MUST BE YES |
| Is the Patient consented or are you treating in best interests | MUST BE YES |
Do not proceed with thrombolysis if any of the above answers are NO - take advice if unsure.
ALTEPLASE
Alteplase comes as a vial with powder and water for reconstitution and diluent. Please see the body-weight chart in the appendix of this policy.
ALTEPLASE Total dose: 0.9mg/kg. MAXIMUM DOSE IS 90 MG. Should be prescribed by and administration supervised by a doctor (Registrar or above) once the approval has been obtained from the Stroke consultant. Give 10% of total dose given as an IV push over 2 minutes. Give remaining 90% of dose IV over 60 minutes via infusion pump.
After giving Alteplase
Procedures to Avoid Post Thrombolysis as these may cause trauma and mucosal or intramuscular bleeding and may well be unnecessary anyhow. In the first 24 hours immediately after stroke thrombolysis : Avoid
- Nasogastric tube unless absolutely necessary.
- Urinary Catheter unless absolutely necessary (retention)
- IM injections and Central line insertion
Avoid All antithrombotic medications. Can use Paracetamol IV/Oral/PR for analgesia or pyrexia
Monitoring
Patients must be continuously monitored following Alteplase and for at least 24 hours following administration for potential side effects and complications. Good blood pressure control is required post-thrombolysis to reduce the potential risk of haemorrhagic transformation. It is vital to reduce it carefully to below the threshold of 185/110 mmHg. Do not strive for a normal BP. Rapid reductions in blood pressure can reduce brain perfusion and worsen outcome. The normal cerebral autoregulation of blood flow is impaired during the acute stroke and blood flow can be greatly affected by rapid systemic changes.
Blood pressure Management
- Monitor blood pressure for the first 24 hours after starting treatment
- Every 15 minutes for 2 hours after starting the infusion.
- Every 30 minutes for 6 hours
- Every hour for 18 hours.
If BP > 185/110 mmHg first check for usual causes e.g. pain, urinary retention. Consider either IV labetolol 20 mg over 1 to 2 minutes.The dose may be repeated and/or doubled every 10 minutes. A dose of up to 150 mg may be given. IV Sodium Nitroprusside (0.5 to 10 mg/kg/min). Nitrate patches may be used. May be combined with oral therapy such as Amlodipine 5 mg stat dose. Use local protocols.In all cases continue agent until BP < 185/110 mmHg is reached. Monitor blood pressure every 15 minutes during the antihypertensive therapy.
Keep BP safely below 185/110 mmHg during and after thrombolysis. This needs actively and continuously managed.
Life threatening Complications of Thrombolysis
| Complication | Clinical | Management |
|---|---|---|
| Acute Anaphylaxis | Suspect an anaphylactic reaction if the patient develops: rash. urticaria. bronchospasm, orolingual angioedema (face. lips and tongue swelling alone) hypotension, stridor, shock. | Stop ALTEPLASE. Urgent medical assessment - airway. breathing and circulation. Adrenaline (EPINEPHRINE) 0.5-1 ml 1 in 1000 im or sc NOT iv if anaphylaxis (dose depends on severity of reaction - (Adrenaline is not usually required if isolated orolingual angioedema. This is commoner in those on an ACEI). HYDROCORTISONE 200mg iv. CHLORPHENAMINE 10mg iv. Fluid challenge with IV N-Saline if shocked and consider repeat doses of Adrenaline [US EPINEPHRINE] as required. Involve anaesthetist immediately if orolingual angioedema is threatening airway. |
| Hemiangioneurotic Oedema 1-5% of patients. | Suspect if the patient develops: orolingual angioedema usually affects side of clinical stroke. (face. lips and tongue swelling alone) hypotension. It may overlap with anaphylaxis and treatment is similar. stridor, shock. Commoner in those on ACEI inhibitors and those of Afro-carribean origin. | Urgent medical assessment - airway. breathing and circulation. Stop Alteplase. Lay flat. Adrenaline (EPINEPHRINE) 0.5-1 ml 1 in 1000 im into anterolateral thigh but never iv if anaphylaxis (dose depends on severity of reaction - (Adrenaline is not usually required if isolated orolingual angioedema. This is commoner in those on an ACEI). HYDROCORTISONE 200mg iv. CHLORPHENAMINE 10mg iv. Fluid resuscitation IV N-Saline 500ml-1L fluid challenge. Consider repeat doses of Adrenaline [US EPINEPHRINE] as required. Involve anaesthetist immediately if orolingual angioedema is threatening airway. Tongue swelling may be due to angioneurotic oedema or bleeding and haematoma. If unsure as management differs then CT of the tongue can distinguish haematoma from angioedema in this setting |
| Symptomatic intracranial haemorrhage 4-6% of patients. | There is a Fall in GCS. a new headache. Seizure. Increasing NIH score. Acute hypertension. Nausea and vomiting. | Immediately Stop ALTEPLASE. Arrange a CT scan. Check PT. APTT. platelet count. fibrinogen. and type and cross-match if not already done so. Haematology advice is that Fibrinolysis will usually result in a low fibrinogen which can be replaced by one pool of cryoprecipitate. If the patient has received antiplatelet therapy such as ASPIRIN then a platelet transfusion should also be considered. Liaise with haematology. Discuss with neurosurgeons and document advice though surgical management would be unusual in this situation until coagulation state normalised. Consider second CT scan to assess the progression of intracranial haemorrhage. Inform Family and discuss prognosis which is usually very poor depending on the extent of bleeding |
| Extracranial haemorrhage | Suspect extracranial haemorrhage if there is a drop in BP, tachycardia, shock, epistaxis, melaena, haematuria, haematemesis, abdominal pain and bruising or pain in flanks or thighs. | Immediately stop infusion of ALTEPLASE. Use direct pressure if possible. to control bleeding from arterial or venous puncture sites. Check fibrinogen, PT, APTT, full blood count and arrange an appropriate cross match and urgent transfusion to match blood losses. Support circulation with fluids and blood transfusion as appropriate. Haematology advice is that if bleeding has been confirmed by imaging that blood should be taken for Group and save. FBC and coagulation screen. Fibrinolysis will usually result in a low fibrinogen level which can be replaced by one pool of cryoprecipitate. If the patient has received antiplatelet therapy such as ASPIRIN then a platelet transfusion should also be considered. Liaise with haematology. Consider transfusion of fresh frozen plasma and/or cryoprecipitate depending upon the results of a coagulation screen. Appropriate referral e.g. ENT for persisting epistaxis. Gastroenterology for melaena, haematemesis etc |
Comments from the ALTEPLASE datasheet - take haematology advice If a potentially dangerous haemorrhage occurs in particular cerebral haemorrhage. The fibrinolytic therapy must be discontinued. In general. however. it is not necessary to replace the coagulation factors because of the short half-life and the minimal effect on the systemic coagulation factors. Most patients who have bleeding can be managed by interruption of thrombolytic and anticoagulant therapy. volume replacement. and manual pressure applied to an incompetent vessel. Protamine should be considered if heparin has been administered within 4 hours of the onset of bleeding. In the few patients who fail to respond to these conservative measures. judicious use of transfusion products may be indicated. Transfusion of cryoprecipitate. fresh frozen plasma. and platelets should be considered with clinical and laboratory reassessment after each administration. A target fibrinogen level of 1 g/l is desirable with cryoprecipitate infusion.
Improving Stroke to Needle Time for earlier Thrombolysis
Those who do best with thrombolysis are those treated within the first 90 minutes of stroke. After that is a typical decay curve in terms of ongoing gain until it reaches no gain at about 4.5 hours. The fact that patients can be treated out to this time is no excuse whatsoever for any delays in arrival, assessment, scanning and treatment. It is vital that times are audited for all cases and action plans and indeed root cause analysis for any significant delays or breaches. Certainly a door to needle time of 1 hour should be the limit and 45 minutes the ideal target to allow proper assessment and scanning. If unsure what to do then it is better to take advice from someone more experienced so a decision can be made. Would also caution against assuming that it is a TIA and is getting better unless there is complete resolution within 10-20 minutes. Anything longer then assess and treat as stroke.
Potential Barriers
Stroke to Door
- Patient not recognising symptoms of stroke (delay seeking help)
- Patient unable to access help
- Family/Carers not recognising symptoms of stroke (delay seeking help)
- Patient or family calling General practitioner (GP) first
- GP receptionists not recognising potential stroke
- Paramedics not realising stroke and failure to pre-alert
Door to Needle
- Incorrect triage in ED
- Delays in requesting, portering, access to CT (in hours, out of hours)
- Delays in following in-hospital pathways
- Delays in stroke team arriving
- Delay in obtaining consent
- Waiting to see if things improve
- Decisional inertia by ED ? TIA
- Telemedicine access (who is on and who to call, software, hardware)
- Physician unfamiliarity with recombinant tissue plasminogen activator (rt-PA) use
- Nursing unfamiliarity with protocols, pathways and drugs
Patients with suspected stroke should have
- Blue light Ambulance in appropriate cases
- Hospital Pre-alert
- Rapid triage on arrival at hospital
- Immediate access to specialist stroke services
- Immediate and rapid brain imaging
- Rapid specialist assessment
Other possible interventions
- Education programmes to improve the general public recognition of symptoms of stroke
- Training paramedics to diagnose stroke more accurately and decrease time to hospital
- Helicopter transfer where appropriate
- Training emergency medical staff in acute stroke care
- Reorganisation of hospital systems
- Multifaceted interventions (including telemedicine systems)
Telemedicine
A systematic review of five observational studies concluded that telemedicine systems can be feasible, acceptable and technically and diagnostically reliable in acute stroke management, and that telemedicine consultations were associated with improved delivery of rt-PA. In areas without a local stroke specialist, telemedicine consultation should be considered to facilitate treatment in patients eligible for thrombolysis (Grade B)
Monitoring and Audit and Clinical Governance
It was agreed that all thrombolysis given should be registered on an international SITS database based in Stockholm. Stroke units in the UK should adhere to this and also thrombolysis is recorded on the SSNAP database. This records age, stroke type and the occurrence of any secondary haemorrhage. It is useful for all centres to record this data and al stroke centres should be auditing their rates of thrombolysis, doing analysis if those who have delayed lysis and looking at those who failed to be given lysis without a clear contraindication at frequent M&M meetings as part of good clinical governance.
References and further reading
- Röther J1, Ford GA, Thijs VN. Cerebrovasc Dis. 2013;35(4):313-9. Thrombolytics in acute ischaemic stroke: historical perspective and future opportunities.
- Tissue plasminogen activator for acute ischemic stroke: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333: 1581-1587
- Intravenous Thrombolysis With Recombinant Tissue Plasminogen Activator for Acute Hemispheric Stroke The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274(13):1017-1025
- Thrombolysis for acute ischaemic stroke. Joanna M Wardlaw, Veronica Murray, Eivind Berge, and Gregory J del Zoppo. Cochrane Database Syst Rev. ; 7:
| Note: The plan is to keep the website free through donations and advertisers that do not present any conflicts of interest. I am keen to advertise courses and conferences. If you have found the site useful or have any constructive comments please write to me at drokane (at) gmail.com. I keep a list of patrons to whom I am indebted who have contributed. If you would like to advertise a course or conference then please contact me directly for costs and to discuss a sponsored link from this site. |